The Guide to Pharmaceutical Warehouse Requirements

Whether you're a pharmaceutical manufacturer or a distributor, understanding and adhering to pharmaceutical warehouse requirements is crucial to ensure product safety and efficacy.
A pharmaceutical warehouse has many regulations to comply with, primarily focused on ensuring the quality, integrity, and identity of products being stored. This means that consideration must be given to a warehouse’s layout and temperature control, and maintaining detailed documentation to demonstrate a product’s quality, identity, and chain of custody.
This guide will discuss essential pharmaceutical warehousing requirements, including regulatory compliance, layout considerations, and best practices.
An Overview of GMP and GWP
Good Manufacturing Practices (GMP) and Good Warehouse Practices (GWP) are fundamental principles in the pharmaceutical industry. GMP ensures that pharmaceutical products are consistently produced according to quality standards. GWP, on the other hand, focuses on maintaining the integrity of products during storage and distribution.
GMP includes rigorous quality control measures, while GWP emphasizes the importance of proper warehousing techniques. Both are vital for ensuring pharmaceutical products remain safe and effective throughout their lifecycle.
The Layout of a Pharmaceutical Warehouse
Efficient warehouse layout is crucial for pharmaceutical companies. Properly organized warehouses streamline operations and reduce the risk of errors. Considerations include the placement of products, picking routes, and the use of storage equipment like shelving and racking systems.
Pharmaceutical warehouses are typically laid out with separate storage areas based on each product’s current needs. The exact areas may vary depending on inventory and its requirements, but a typical warehouse will contain the following areas:
- A cold room. The cold room maintains a temperature between 2°C and 8°C, which is the most common storage temperature range for ensuring the stability of pharmaceutical products. This area usually contains a separate section for quarantined products (products that have not yet been inspected).
- A quarantine area. Incoming products that have not yet been inspected by the quality team must be quarantined separately until their condition is ascertained—usually by checking datalogger reports.
- A released area. Products that have passed inspection and are ready for dispatch are stored here.
- A rejected area. Batches that have failed inspection and have been rejected are separated here.
- A recalled area. Recalled products are kept here.
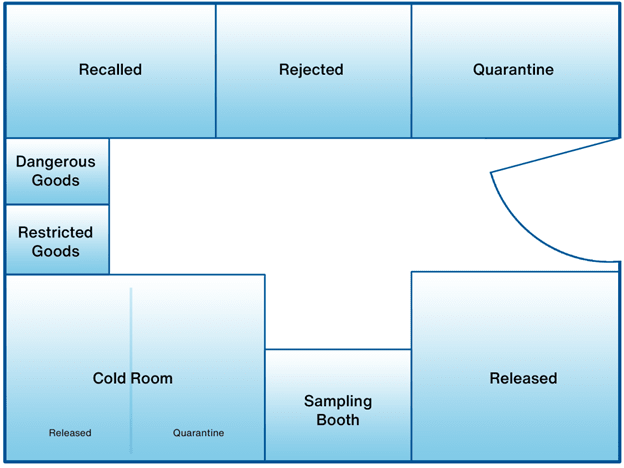
Additionally, some warehouses may include:
- Locked area. Only authorized staff can access this area, where restricted products are stored.
- Sampling room. A room for testing starting chemicals. It will be sealed from the rest of the warehouse in order to protect the testing environment.
- Dangerous goods storage area. Dangerous goods are stored here, usually with special storage conditions.
Regulations for Pharmaceutical Warehouses
Compliance with regulatory standards is an important pharmaceutical warehouse requirement. Here are some key regulations and guidelines:
21 CFR Part 210/211
These regulations set the standard for Current Good Manufacturing Practices (cGMP) for pharmaceuticals. They cover various aspects of drug production, including storage and distribution, and are designed to ensure the safety, identity, quality, and safety of all drugs, regardless of whether they are for prescription use or available over the counter. Note that these regulations also apply to drugs for administration to animals.
21 CFR Part 210 concerns the manufacturing, processing, packing, and holding of drugs, while 21 CFR Part 211 concerns finished pharmaceuticals. Compliance with these regulations is essential to ensure product quality and patient safety.
USP <1079>
Chapter <1079> of the United States Pharmacopeia (USP) outlines comprehensive guidelines for the proper storage and shipping practices that pharmaceutical companies must follow. These guidelines are indispensable for maintaining the integrity of pharmaceutical products from the moment they are manufactured until they reach the end-user, be it a patient or a healthcare facility.
The risk-based guidance includes best practices, including proper temperature control, documentation, training, risk management, and storage conditions. These pharmaceutical warehouse requirements should be closely followed to ensure a compliant facility that presents minimal risk to product quality.
USP <659>
USP <659> is a crucial chapter of the United States Pharmacopeia (USP) that offers comprehensive guidance on temperature management for pharmaceutical products. This chapter outlines best practices for maintaining proper temperature conditions during both storage and transportation. Familiarity with USP <659> is essential for pharmaceutical warehouse compliance.
This chapter not only defines temperature ranges and specifications for refrigeration equipment but also establishes requirements for temperature monitoring and record-keeping. Adherence to these guidelines is necessary to both ensure and demonstrate effective temperature control within a pharmaceutical warehouse.
Title 21 Part 600
Title 21 Part 600 of the Code of Federal Regulations concerns the storage and distribution requirements for biological products. This chapter establishes guidelines to ensure that biological products—including vaccines, blood, and blood components, allergenics, and other biologically derived pharmaceuticals—remain safe and effective between manufacture and administration. These regulations address various aspects, including storage conditions, quality control, labeling, and documentation.
Prescription Drug Marketing Act (PDMA)
The Prescription Drug Marketing Act (PDMA) is a significant piece of United States legislation for ensuring the integrity and safety of the pharmaceutical supply chain. Enacted to combat the growing concern of counterfeit drugs infiltrating the legitimate market, the PDMA imposes stringent requirements on drug wholesalers.
Wholesalers are required to provide a product’s pedigree prior to distribution, verifying that a drug is authentic and has not been tampered with. Record-keeping (maintaining records of drugs' sources, purchases, sales, and returns) and licensing (licensing wholesalers to distribute pharmaceuticals) help to reduce risk of counterfeit drugs, while secure storage and handling ensures that a drug maintains its integrity and efficacy.
State Boards of Pharmacy
Individual State Boards of Pharmacy do not have a uniform set of regulations. State-level pharmaceutical warehouse requirements can vary, particularly concerning temperature control, and some states have very limited rules. It is important to remain aware of state-specific regulations, and to always make protecting product quality a priority.
This is a changing landscape, however. Many state boards of pharmacy are adopting new rules, placing a greater emphasis on temperature monitoring. An early adopter of such rules was Oregon. Since 2016, Oregon’s state board of pharmacy has required automated temperature data logging, maintenance of temperature records, and continuous monitoring of refrigerators. Georgia and Oklahoma have also followed suit. As states continue to update their rules, manual temperature monitoring, and the risks it poses to product quality, is increasingly frowned upon.
Best Practices for Complying with Pharmaceutical Warehouse Requirements
Pharmaceutical warehouses must keep product quality and patient safety at the center of the operational processes. Here are a few best practices than can help to maintain compliance and preserve product integrity.
Store Products at the Correct Temperature
Proper temperature control is critical in protecting the quality of pharmaceuticals, and for complying with regulations. Pharmaceutical products often have strict temperature requirements, and deviations can compromise their efficacy.
As well as ensuring that refrigeration equipment is set to the correct temperatures, it is critical to implement accurate, compliant temperature monitoring systems to be confident that the correct temperatures were maintained during storage. This is a regulatory requirement.
Complete Visibility into the Supply Chain Process
Maintaining transparency in the supply chain helps prevent product spoilage and recalls. Stock should be segregated into separate areas for usable, recalled, expired, and returned products. All stock should be kept in its packaging, clearly displaying its temperature requirements and expiration dates. It is also best practice to maintain digital documentation of all product incomings and outgoings, and their respective temperature requirements, to avoid product risk and help you in the event of an audit.
Maintain a Secure Environment
Security is crucial to safeguard pharmaceutical products from theft or tampering. Certain products, such as dangerous products or controlled substances, should be stored separately with limited access. Access control systems and surveillance can enhance security measures.
Digital security is also critical. Digital data storage must comply with US FDA 21 CFR Part 11 and EU GDP Annex 11, which ensure validity of digital record keeping. Data must be stored in a secure system of record, where it can’t be altered or tampered with. Access to this data should only be provided to the people who require access.
Identify Risk Areas
Warehouses can have temperature variations within different zones. Identifying these areas in advance allows for proactive temperature management and risk mitigation.
A thermal mapping study will identify the areas in which temperatures deviate from the set temperature, allowing actions and procedures to mitigate the effect. In some cases, a thorough study will also identify the causes of these deviations and suggest process improvements to prevent future risks to product quality.
Identifying risk areas and conducting thermal mapping studies are pharmaceutical warehouse requirements under USP <1079>. Any significant risks identified during this process should be addressed, and a new risk study undertaken.
Final Thoughts
In the highly regulated world of pharmaceuticals, adherence to warehouse requirements is non-negotiable. Failure to comply can result in regulatory penalties, product recalls, and damage to a company's reputation. By following GMP, GWP, and other regulations while implementing best practices, pharmaceutical warehouses can operate efficiently and ensure the safety and efficacy of the products they store and distribute.
Our professional services team can provide you with guidance and services to help with thermal mapping services and sensor placement analysis to ensure your pharmaceutical warehouse remains compliant and effective. Contact Us today to connect with a representative from one of our global offices.